It is an electrochemical process that thickens and toughens the naturally occurring protective oxide. The resulting finish, depending on the process, is the second hardest substance known to man, second only to the diamond. The anodic coating is part of the metal, but has a porous structure which allows secondary infusions, (i.e. organic and inorganic coloring, lubricity aids, etc.).
- Anodizing is an electrochemical process that produces thick, nonconductive and tough, second only to diamond, protective oxide coating.
- Porous structure allows to infuse organic, inorganic colors and PTFE (Teflon) based solid film lubricants.
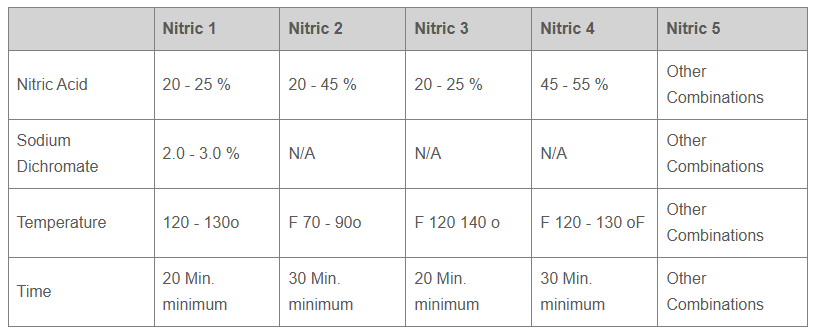
- Anodizing is also referred to as anodic coating and commercially achieved by using sulfuric acid and chromic acid electrolytes.
- Chromic acid anodizing is used to produce thinner corrosion protective oxide coating.
- Chromic acid anodizing is specified mostly for welded and porous cast aluminum structures in the aerospace application as trace chromic acid left during process does not corrode the parts.
- Sulfuric acid anodizing process is used to produce corrosion protective oxide coating for most industrial and decorative applications. Sulfuric acid anodized coating dyes easily producing wide varieties of colors.
- Hard coat anodize is produced at low temperature and high current density using sulfuric acid with special additives. Hard coat anodize is used to produce thick oxide coating, generally 2 mil or 50 Micron oxide coating for mostly abrasion/wear resistant, high heat dissipation and dielectric properties.
- All the anodic coating can be dyed to various exotic colors.
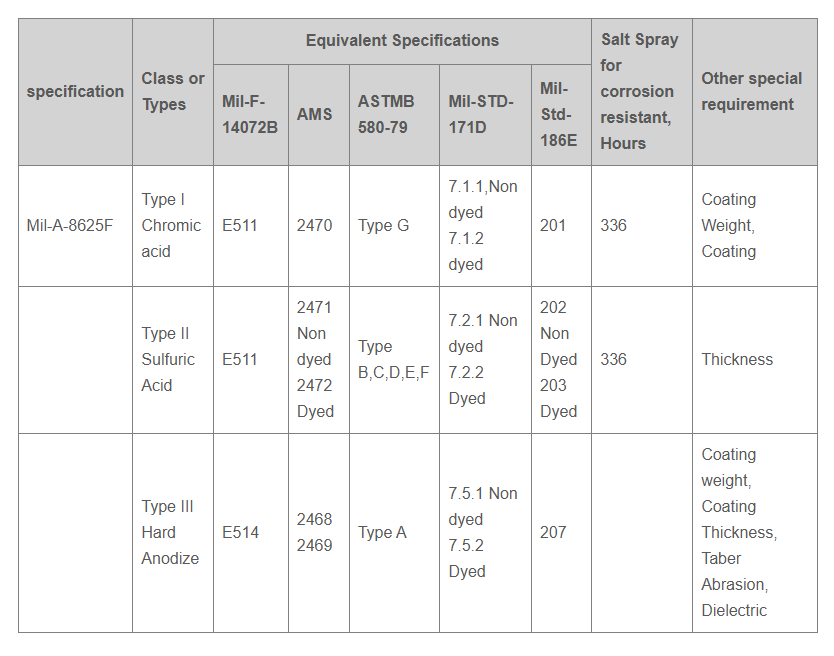
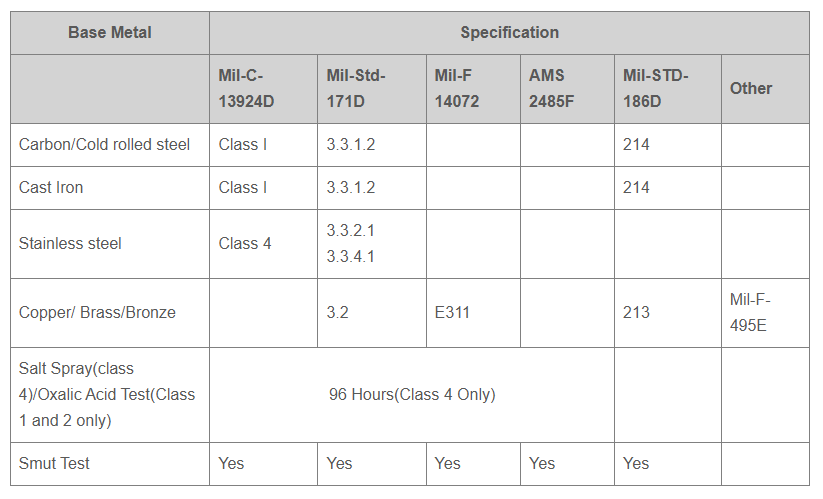
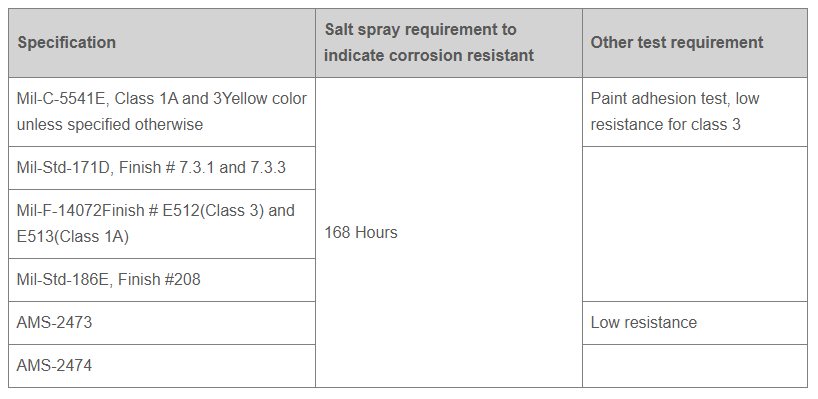
- Anodic coating is covered by various Military, ASTM, AMS, Federal and customer specifications.
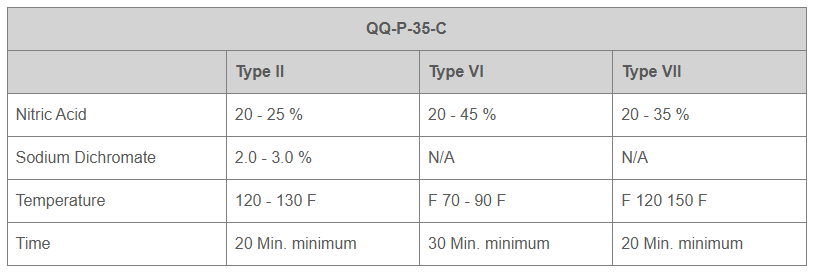
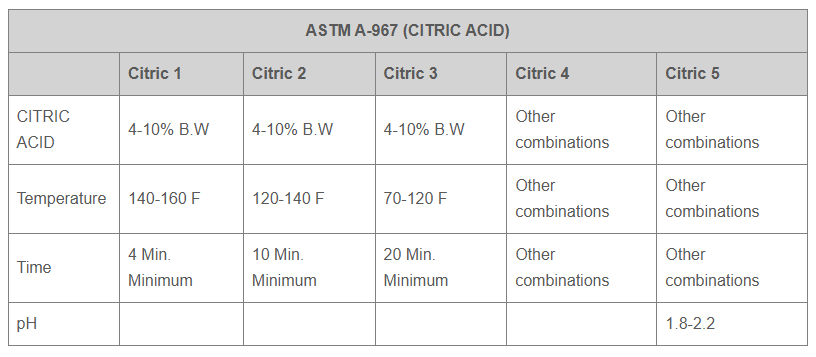
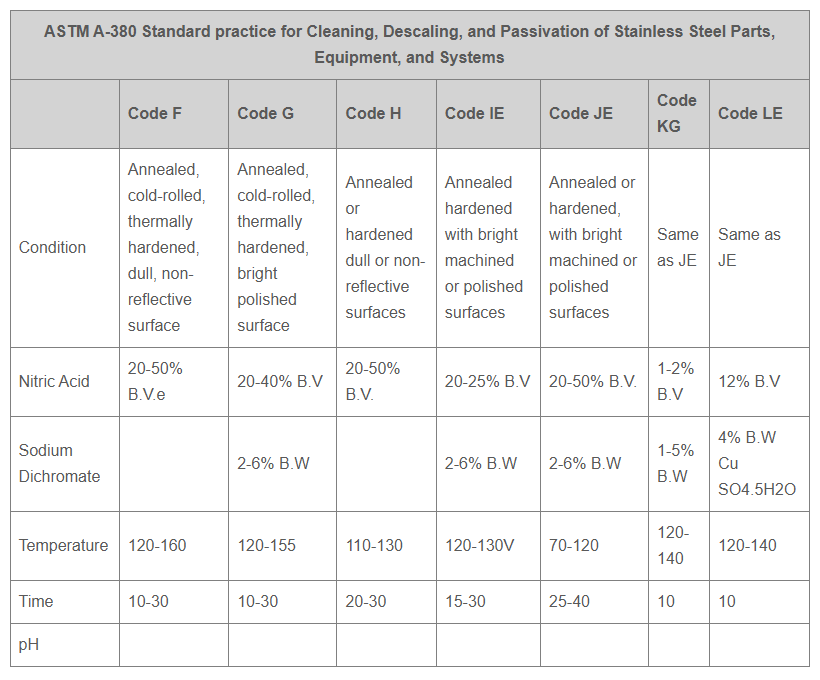
Andarn has the capability to perform and certify finishing to the following specifications: